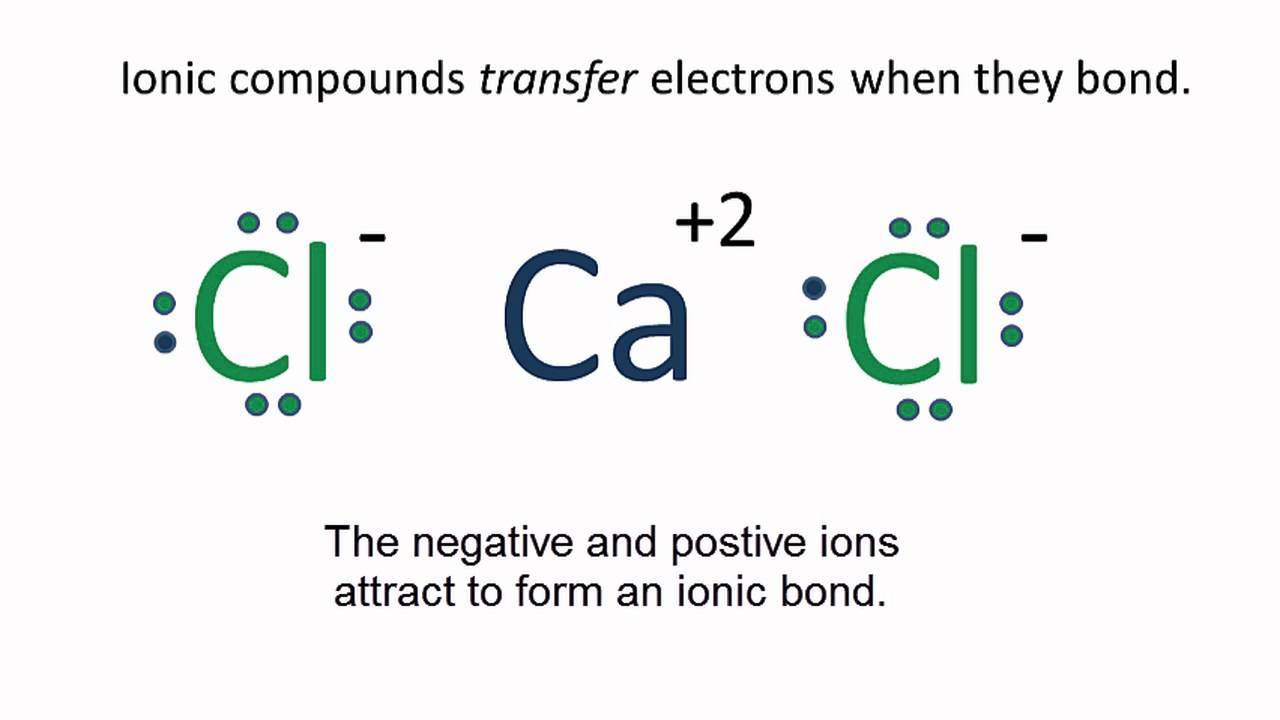
Lewis Structure Worksheet And Key
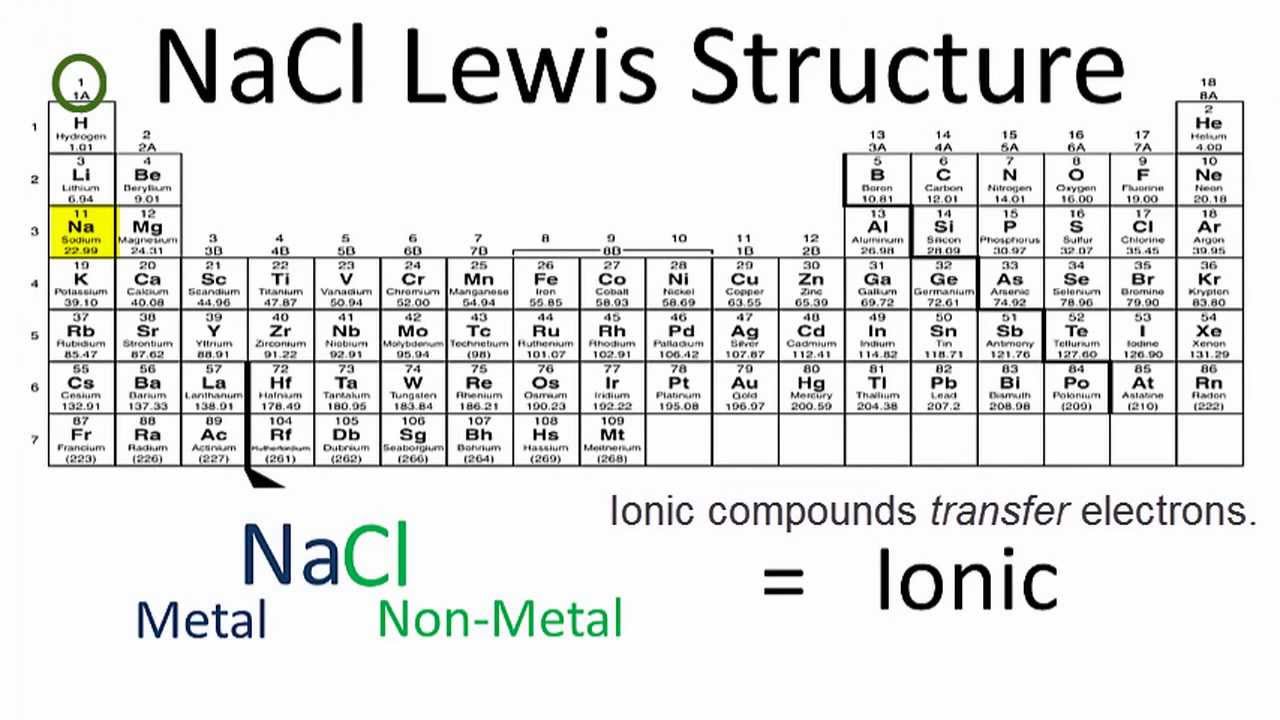

Regents Chemistry Topics Crazy mohan drama mp4 free. | Unit 5 Bonding | | TEDED Bonding Video | Bond Polarity | Dipole Dipole Forces | | Types of Bonds | Properties of Covalent Compounds | Hydrogen Bonding | | Ionization Energy | Network Solids | van der Waals Forces | | Lewis Dot Structures-Covalent | Metallic Bonds | Like Dissolves Like | | 70 Lewis Dot Structures Videos | (AP) Valence Shell Electron Pair Repulsion VSEPR(Shapes) | | Lewis Dot Structures -Ionic | Molecular polarity | Percent Water in a Hydrate | | Properties of Ionic Compounds | Intermolecular Force |
Advanced Regents Chemistry Topics | Unit 5 Bonding | | TEDED Bonding Video | (AP) Bond Energy | Hydrogen Bonding | | Types of Bonds | Properties of Ionic Compounds | van der Waals Forces | | Ionization Energy | Properties of Covalent Compounds | Like Dissolves Like | | (AP) Electron Affinity | Network Solids | | Lewis Dot Structures-Covalent | Metallic Bonds | Percent Water in a Hydrate | | (AP) Formal Charge | (AP) Valence Shell Electron Pair Repulsion VSEPR(Shapes) (AP) Bond Angles | | 70 Lewis Dot Structures Videos | (AP) Hybridization of Orbitals (AP) Determining Hybridization in Structures | (AP)Valence Bond Theory | | Lewis Dot Structures -Ionic | Molecular polarity | | (AP)Sigma and Pi Bonds | Intermolecular Force | | Bond Polarity | Dipole Dipole Forces |
Moshi monsters 500 rox codes.My Chemical Demonstration Videos |
|
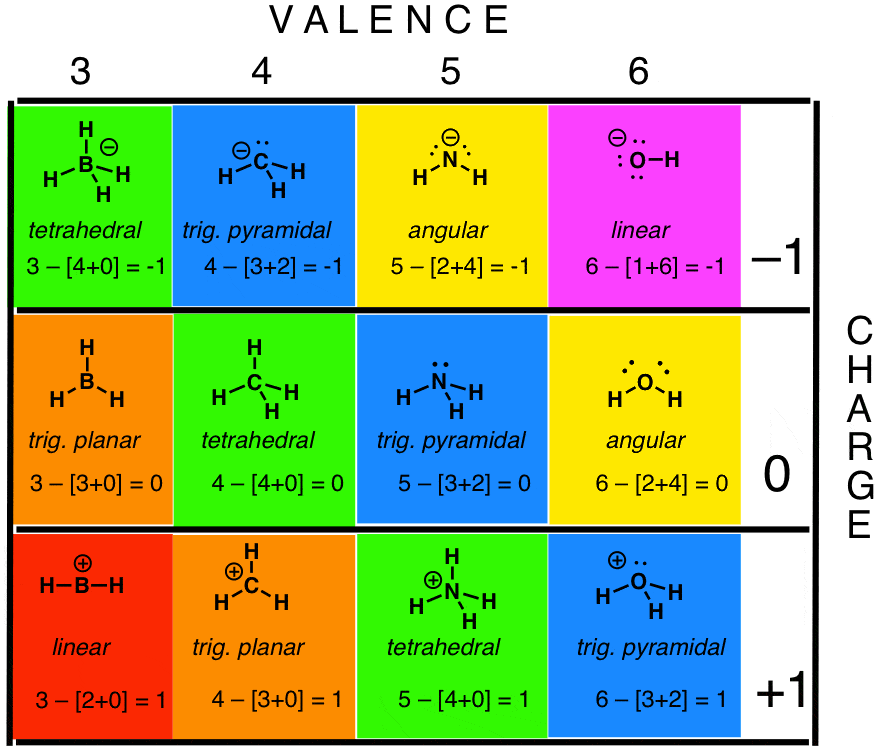
Lewis Dot Structure Practice
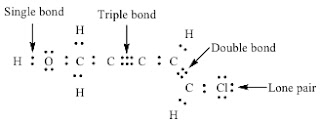
Lewis Dot Structure Calculator With Dots
It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds. This suggests the best Lewis structure has three B–F single bonds and an electron deficient boron. There are three basic steps to determining the bond angles in a molecule: 1. Write the Lewis dot structure for the molecule. Assume that you must determine the bond angles in 'BF'3. 'B' is less electronegative than 'F', so 'B' becomes the central atom. If we have three 'F' atoms, that means that we are going to use all three electrons from the 'B'. This gives us three bonding pairs of. Given descriptions, diagrams, scenarios, or chemical symbols, students will model covalent bonds using electron dot formula (Lewis structures). Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Quiz your students on PCl4+ Lewis Dot Structure - Bond Angle, Hybridization, Molecular Geometry using our fun classroom quiz game Quizalize and personalize your teaching.